You Don’t Have To Live With Joint Pain
Total Hip Replacement (Continued)
You Don’t Have To Live With Severe Hip Pain
The Trident® Ceramic Acetabular System
The Trident® Ceramic Acetabular System (Continued)
You Don’t Have To Live With Joint Pain
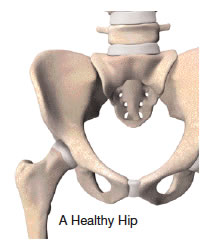 Your joints are involved in almost every activity you do. Simple movements such as walking, bending, and turning require the use of your hip and knee joints. Normally, all parts of these joints work together and the joint moves easily without pain. But when the joint becomes diseased or injured, the resulting pain can severely limit your ability to move and work. Osteoarthritis, one of the most common forms of degenerative joint disease, affects an estimated 43 million people in the United States.1 Whether you are considering a total joint replacement, or are just beginning to explore available treatments, this website is for you. It will help you understand the causes of joint pain and treatment options. Most importantly, it will give you hope that you may be able to return to your favorite activities.
Your joints are involved in almost every activity you do. Simple movements such as walking, bending, and turning require the use of your hip and knee joints. Normally, all parts of these joints work together and the joint moves easily without pain. But when the joint becomes diseased or injured, the resulting pain can severely limit your ability to move and work. Osteoarthritis, one of the most common forms of degenerative joint disease, affects an estimated 43 million people in the United States.1 Whether you are considering a total joint replacement, or are just beginning to explore available treatments, this website is for you. It will help you understand the causes of joint pain and treatment options. Most importantly, it will give you hope that you may be able to return to your favorite activities.
Once you’re through reading this website, be sure to ask your doctor any questions you may have. Gaining as much knowledge as possible will help you choose the best course of treatment to relieve your joint pain — and get you back into the swing of things.
References:
1. Arthritis Foundation website, Feb. 2006.
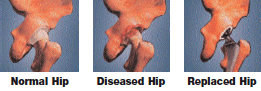
What Is A Hip Joint?
Your hip joint is a ball-and-socket joint, formed by the ball, or femoral head, at the upper end of the thighbone, and the rounded socket, or acetabulum, in the pelvis. The bone ends of a joint are covered with a smooth, tough material called cartilage. Normal cartilage cushions the bones and allows nearly frictionless and pain-free movement. The rest of the surfaces of the joint are covered by a thin, smooth tissue lining called the synovium. The synovium produces fluid that acts as a lubricant to reduce friction and wear in the joint.
Common Causes Of Joint Pain
 Osteoarthritis (OA)
Osteoarthritis (OA)
Sometimes called degenerative arthritis because it is a “wearing out” condition involving the breakdown of cartilage and bones. When cartilage wears away, the bones rub against each other, causing pain and stiffness. OA usually occurs in people aged 50 years and older, and frequently in individuals with a family history of arthritis.
Rheumatoid Arthritis (RA)
Causes the synovium to become thickened and inflamed. In turn, too much synovial fluid is produced within the joint space, which causes a chronic inflammation that damages the cartilage. This results in cartilage loss, pain, and stiffness. RA affects women about 3 times more often than men, and may affect other organs of the body.
Post-traumatic Arthritis
May develop after an injury to the joint in which the bone and cartilage do not heal properly. The joint is no longer smooth and these irregularities lead to more wear on the joint.
Avascular Necrosis
Can result when bone is deprived of its normal blood supply. Without proper nutrition from the blood, the bone’s structure weakens and may collapse and damage the cartilage.
Paget’s Disease
A bone disease that often affects the hip. Bone formation is sped up, causing the density and shape of the bone to change. Joint pain can also be caused by deformity or direct injury to the joint. In some cases, joint pain is made worse by the fact that a person will avoid using a painful joint, weakening the muscles and making the joint even more difficult to move.
Treatment Options
Following the orthopaedic evaluation, your orthopaedic surgeon will review and discuss the results with you. Based on his or her diagnosis, your treatment options may include:
- Medication
- Joint fluid supplements
- Physical therapy
- Joint replacement
Total Hip Replacement
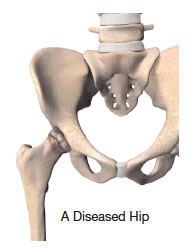
 Hip replacement is one of the most important surgical advances of this century. This surgery helps more than 300,000 Americans each year1 to relieve their pain, and get back to enjoying normal, everyday activities. Hip replacement involves the removal of arthritic bone ends and damaged cartilage and replacing them with prosthetic implants that replicate the hip joint.
Hip replacement is one of the most important surgical advances of this century. This surgery helps more than 300,000 Americans each year1 to relieve their pain, and get back to enjoying normal, everyday activities. Hip replacement involves the removal of arthritic bone ends and damaged cartilage and replacing them with prosthetic implants that replicate the hip joint.
Hip replacement surgery may be considered when arthritis limits your everyday activities such as walking and bending, when pain continues while resting, or stiffness in your hip limits your ability to move or lift your leg. Hip replacement may be recommended only after careful diagnosis of your joint problem. It is time to consider surgery if you have little pain relief from anti-inflammatory drugs or other treatments, such as physical therapy, do not relieve hip pain. Hip replacement can help relieve pain and get you back to enjoying normal, everyday activities.
Total hip replacement is often reserved for patients who:
- Have a painful, disabling joint disease of the hip resulting from a severe form of arthritis
- Are not likely to achieve satisfactory results from less invasive procedures, such as arthrodesis (artificial stiffening or fixation of the joint)
- Have bone stock that is of poor quality or inadequate for other reconstructive techniques
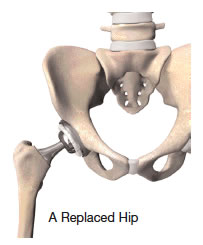
In a total hip replacement operation, the surgeon replaces the worn surfaces of the hip joint with an artificial hip joint. The worn head of the femur (thigh bone) is replaced with a metal or ceramic ball mounted on a stem; the stem is placed firmly into the canal of the thigh bone at its upper end. The acetabulum (hip socket) is prepared and implanted with a metal cup and plastic or ceramic insert. The ball and insert glide together to replicate the hip joint.
References:
1. Orthopedics.about.com website, Feb. 2006.
Total Hip Replacement (Continued)
 Total Hip Implants
Total Hip Implants
The conventional arrangement of a metal ball into a special plastic (polyethylene) cup has been shown to have positive results over the years. How long it will last depends not only on age, but also on a patient’s activity level.
Another factor that may affect the durability of a total hip replacement is the bearing surface. The bearings are the two parts of the artificial hip that glide together throughout motion. These bearings can be metal-on-polyethylene, metal-on-metal, ceramic-on-polyethylene or ceramic-on-ceramic.
Technologies That May Impact Implant Performance
There have been significant advancements in improving the bearing surfaces in total hip replacement. Ceramic-on-ceramic bearings provide superior wear performance.1 Stryker’s ceramic-on-ceramic system has demonstrated significantly lower wear than metal-on-polyethylene systems in the laboratory; therefore, it is anticipated that these improved wear characteristics may extend the life of the implant.
There are also new, advanced polyethylene implants available that have demonstrated extremely low wear in the laboratory, and they are expected, over time, to have similar wear performance clinically.2
Your physician will discuss the exact type of prosthesis and surgical procedure with you.
Complications of Hip Replacement
As with any surgery, there is risk of complications after hip replacement surgery. However, they are relatively rare. Blood clots are the most common complication after surgery. Your orthopaedic specialist may prescribe one or more measures to prevent a clot from forming in your leg veins. These measures may include special support hose, inflatable leg coverings and blood thinners.
Stryker Corporation or its divisions or other corporate affiliated entities own, use or have applied for the following trademarks or service marks: Accolade, Stryker, Trident and TMZF. All other trademarks are trademarks of their respective owners or holders.
References:
1. Taylor. S.K., Serekian, P., Manley, M., “Wear Performance of a Contemporary Alumina: Alumina Bearing Couple under Hip Joint Simulation,” Trans. 44th Ann. Mtg. ORS, 1998.
2. Stryker Test Report RD–04–099.
Recovery
Generally, after hip replacement surgery, you may spend approximately 3 to 5 days in the hospital. Most hip replacement patients begin standing and walking with the help of a walker and a physical therapist the day after surgery.
Recovery varies with each person. It is essential that you follow your orthopaedic surgeon’s instructions regarding home care during the first few weeks after surgery; especially the exercise program you are prescribed. You should be able to resume many normal light activities of daily living within 3 to 6 weeks following surgery. Some discomfort with activity, and at night, is common for several weeks. Complete recovery can take from about 3 to 6 months.
While most people will gradually increase their activities and play golf, doubles tennis, shuffleboard or bowling, you will be advised to avoid more active sports, such as jogging, singles tennis and other high impact activities.
You Don’t Have To Live With Severe Hip Pain
You don’t have to live with severe hip pain and the limitations it puts on your activities. If you haven’t experienced adequate relief with medication and other conservative treatments, Hip Joint Replacement may provide the pain relief you long for and enable you to return to your favorite activities. Remember, even if your doctor recommends hip replacement for you, it is still up to you to make the final decision.
For more information contact your doctor.
The Trident® Ceramic Acetabular System
 System Description
System Description
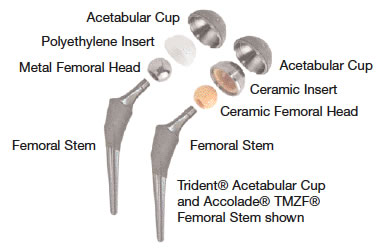
The Trident® Ceramic Acetabular System is an artificial hip replacement device that features a new, state-of-the-art ceramic-on-ceramic bearing couple. The artificial hip replacement device consists of four basic components: an alumina ceramic insert (socket liner), an alumina ceramic femoral head (ball), a metal acetabular shell (socket), and a metal femoral stem (hip stem).
The hip stem is inserted into the top of the thighbone. The ball fits onto the top of the hip stem. The socket liner and mating socket are fixed to the hip joint. The ball and socket articulate together.
The Trident® implant has bearing surfaces (the ball and socket) made of alumina ceramic. Laboratory testing of alumina ceramic has shown it to have less wear than the metal and plastic materials that are currently used in total hip surgery. Alumina ceramic is extremely hard — in fact, its hardness is second only to diamond — and provides excellent lubrication between the ball and socket. Because of its material characteristics, alumina ceramic-on-ceramic demonstrates significantly lower wear versus conventional metal-on-plastic components in laboratory testing. Therefore, it is anticipated that these improved wear characteristics will extend the life of the implants.
Purpose of the Device
This total hip replacement is indicated for patients with painful, disabling hip joint disease caused by a severe form of arthritis. This total hip replacement should not be used for patients:
- Who may have an infection in or near the hip joint
- Who are unable to comply with the instructions for preparing for and recovering from the surgery
- Who do not have enough healthy bone to support fixation of the implants
- Whose bones are not fully grown
Clinical Experience
Howmedica Osteonics Corp. (hereafter referred to as Stryker Orthopaedics) conducted a clinical study in the United States for its ceramic-on-ceramic hip system, which was conducted under a study protocol approved by the United States Food and Drug Administration. The study was performed at 16 orthopaedic centers of excellence across the United States. The clinical study was conducted for Stryker Orthopaedics’ first generation ceramic-on-ceramic design (called the ABC System), and second generation ceramic-on-ceramic design (called the Trident® System). In all, the data from 558 hip replacement surgeries (cases) with these ceramic-on-ceramic bearings were evaluated.
The prospective, randomized, clinical study began in 1996, with 349 cases with the first-generation ceramic-on-ceramic systems, and 165 cases with a control device (conventional, metal-on-polyethylene). The clinical data on these cases were evaluated out to 24 months (2 years) after surgery. In 1999, the patented Trident® Ceramic System was then entered into the study. This patented design offers a stronger, easier-to-use ceramic liner design. Two hundred nine (209) Trident® inserts were implanted in the study. The clinical data from these cases has also been evaluated out to 24 months (2 years) after surgery.
The Trident® System features the identical ceramic-on-ceramic articulating bearing surface as the ABC System; however, the Trident® System features an outer titanium sleeve. The titanium sleeve offers the following unique advantages over other first generation ceramic-on-ceramic designs:
- Increases the strength of the ceramic insert by 50%: The Trident® Alumina Insert is the strongest ceramic insert on the U.S. market today.
- Protects the insert rim from chipping during implant assembly: Occasional chipping of the insert rim during surgery was reported during clinical studies of first-generation designs. No Trident® ceramic implants chipped or fractured in the study.
- Allows reassembly of the acetabular shell to the insert: First generation designs prohibit reassembly of a ceramic insert to its shell, making insert adjustment or replacement more difficult.
Safety Data
Safety of the ceramic-on-ceramic hip systems was established by studying the adverse (unfavorable) events within the clinical study. The adverse events experienced by patients who received the ceramic-on-ceramic hip systems (test group) were comparable to the adverse events experienced by patients who received a conventional, metal-on-polyethylene hip system (control group). There were no deaths in the test or control study groups. Additional surgery to replace or remove components occurred 4 times in the Trident® ceramic-on-ceramic group, as compared to 5 times in the metal-on-poly (control group). Analysis of the adverse event data demonstrated that there was no significant difference between the adverse events experienced in the test and control groups.
Effectiveness Data
The effectiveness of the Trident® ceramic-on-ceramic systems was established by comparing the Harris Hip Scores (HHS) and the radiographic (X-ray) measurements of patients who received the ceramic-on-ceramic systems to those of patients who received the metal-on-polyethylene (control) hip system.
Pain and Function Improvement
The Harris Hip Score is a scale from 1-100 that assesses a patient’s level of pain and function. The highest possible score (100) indicates pain relief and normal functional ability. The lowest possible score (0) indicates severe pain and disability. A score of 90-100 is considered excellent. At two years after surgery, the average Harris Hip Scores for the ceramic-on-ceramic group and for the control group were both in the excellent range.
Stryker Corporation or its divisions or other corporate affiliated entities own, use or have applied for the following trademarks or service marks: Stryker and Trident. All other trademarks are trademarks of their respective owners or holders.
The Trident® Ceramic Acetabular System (Continued)
X-ray (Radiographic) Measurements
X-rays were reviewed at regular intervals after surgery to look for signs of possible device loosening, device movement, or accelerated wearing away of the components. At two years after surgery, all of the 185 Trident® cases evaluated were considered a radiographic success. No devices showed signs of device loosening, device movement, or accelerated wearing away of the components.
Patient Success Rates
A patient was considered a success within the study if, at two years after surgery, the hip implant system was still in place (had not been replaced), the Harris Hip Score was greater than 70 points, and there were no X-ray signs that might indicate loose or unstable hip implant components. The patient success rate for the control group was 94%. The patient success rate for the Trident® ceramic-on-ceramic group was 97%.
Use for Inflammatory Joint Disease
The study results presented above include only patients who had primary total hip replacement for severe, non-inflammatory degenerative joint disease. Eight additional cases of inflammatory joint disease were enrolled in the study, and received a ceramic-on-ceramic system (either ABC or Trident®). The eight cases have been followed for a mean duration of 16 months. As of the latest functional evaluations, the mean HHS is 94 (range 85-100). There have been no reoperations or revisions. There have been no operative hip-related complications. All components appear stable on X-ray.
Potential Benefits
The goals of artificial hip replacement include relief of pain, restoration of function, and correction of deformity. Ceramic-on-ceramic, however, demonstrates significantly lower wear versus conventional metal-on-plastic components in laboratory testing. Therefore, it is anticipated that these improved wear characteristics will result in a longer lasting implant.
Potential Risks
Any artificial hip replacement may be associated with serious complications. These include dislocation, loosening, implant breakage, bone fracture, reaction to the implant’s materials, bone loss, change in the length of the treated leg, pain, hip stiffness, excessive bleeding, hip joint fusion, nerve damage, allergic reaction to medical and/or blood transfusion, infection, reactions to pain relieving drugs, blood clots in the legs and/or lungs, amputation, heart attack, pneumonia, excessive wear of the implant’s components, decrease of bone mass, and audible sounds during motion.
With this ceramic-on-ceramic system, sudden breakage of ceramic components resulting from excessive forces is possible; however, no ceramic component broke during the clinical study. Corrosion (eroding) between the insert and shell may be possible; however, this event was not demonstrated in the clinical study, and laboratory tests have shown the potential to be minimal.
Any of the above-cited complications may require medical intervention, including additional surgery. In rare instances, complications may lead to death. Please ask your surgeon to discuss with you any of these complications that are not familiar to you.
Patient Instruction
Call your doctor if you experience any of the following symptoms:
- Redness, burning, swelling, or drainage from your operated area
- Fever of 100 degrees or higher
- Pain that does not lessen with rest
- Acute, severe pain in the hip associated with twisting, turning or injury
Consult your doctor regarding considerations before surgery, rehabilitation after surgery, and expectations for surgery. It is important to begin planning for your return home from the hospital before your surgical procedure. Your surgeon may suggest tips to prepare your home for after surgery. For example, get an apron or belt with pockets to carry things while you are on crutches, buy or borrow a cordless phone, remove scatter rugs and other obstacles to safe transport using crutches, have high chair and commode accessories available. Above all, during this time, treat yourself well, eat balanced meals, get plenty of rest, and if requested by your surgeon, donate your own blood so it can be transfused during and after surgery.
After surgery you will need to rest your hip to allow proper healing. Your activity will be restricted during this healing period. During the first weeks after surgery, you may be advised to put a pillow between your legs when turning over in bed, wear elastic stockings, use raised toilet seat, take showers rather than baths, restrict activities such as sudden twisting or turning, crossing legs, exposing the scar to sunlight, and driving. Follow carefully your doctor’s instructions regarding progression to normal weight bearing and resumption of normal physical activity. Individual results will vary and all patients will experience different activity levels post-surgery.
Even after the healing period, excessive loads placed on the implants through sudden trauma or high impact activities, such as running and jumping, can damage the artificial joint. While the expected life of an artificial hip replacement system is difficult to estimate, it is finite. The components are made of foreign materials that will not indefinitely withstand the activity level and loads of normal, healthy bone. The hip joint may have to be replaced at some time in the future.
Alternative Treatments
Other options may include use of a conventional hip replacement system, other surgical procedures that do not replace the hip joint, or non-surgical treatments based on pain management and activity restriction. Your doctor can explain these alternatives, and help you decide which treatment is best for your condition.
Stryker Corporation or its divisions or other corporate affiliated entities own, use or have applied for the following trademarks or service marks: Stryker and Trident. All other trademarks are trademarks of their respective owners or holders.